Description
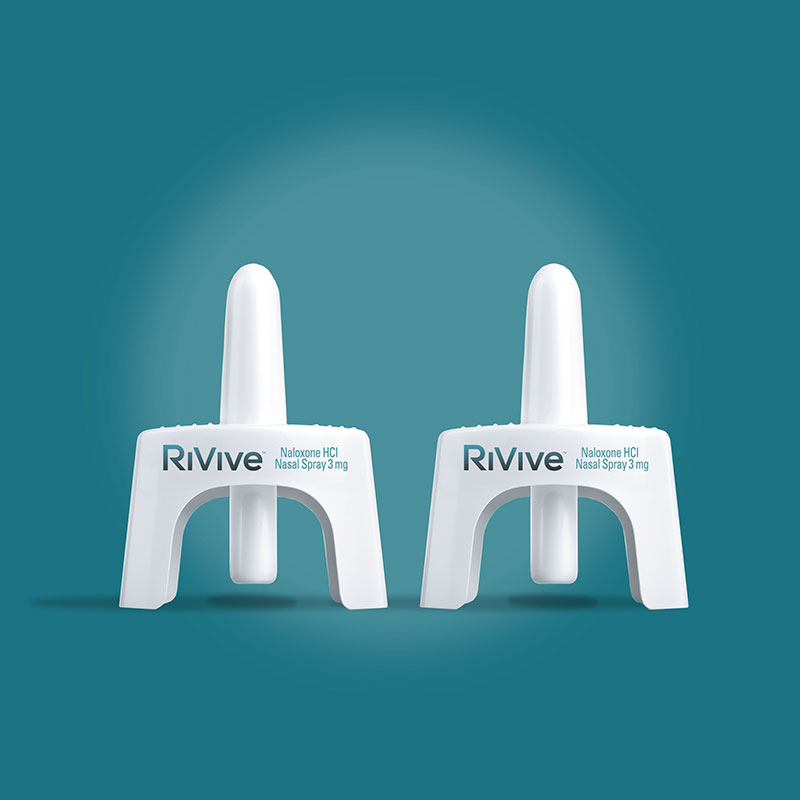
- The carton contains two OTC naloxone hydrocholoride 3 mg nasal spray devices; each packaged in a plastic blister pack with a copy of the directions (quick start guide) and sealed with a paperboard card.
- Each nasal spray device is labeled front and back with the RiVive trade name, lot number, and expiration date.
- The lot number and expiration date are printed on each blister pack and product carton.
- Each corrugated shipping case contains 24 twin pack cartons of RiVive™.
- Each pallet contains 105 corrugated shipping cases (2,520 Twin Pack Cartons).